The potential available nutrients in a soil, whether natural or added in manures or fertilizer, are only in part utilized by plants ...
—T.L. Lyon and E.O. Fippin, 1909
Other Nutrients
Additional nutrient and soil chemical issues remain important, although farmers understandably focus on nitrogen and phosphorus, because additions of these nutrients are commonly needed in order to maintain crop productivity, large quantities are normally used, and both have potential for environmental problems, additional nutrient and soil chemical issues remain important. While K deficiency is also fairly common, most other nutrients are not normally deficient. Micronutrient fertilizers generally are required in cases where the micronutrients are naturally unavailable in the soil, or when many years of intensive crop production has reduced much of the natural soil supply. We focus here mostly on the mineral nutrients that are critical for healthy plants, but some trace elements are also important for animal and human health, including zinc, iron, iodine, calcium, magnesium, selenium and fluorine, which need to be supplied through the food chain (soil-plant-animal/human) or added as nutritional supplements.
Overuse of fertilizers and amendments other than N and P seldom causes problems for the environment, but it may waste money and reduce yields. There are also animal health considerations with excess amounts. For example, excess potassium in feeds for dry cows (cows that are between lactations) results in metabolic problems, and low magnesium availability to dairy or beef cows in early lactation can cause grass tetany. As with most other issues we have discussed, focusing on the management practices that build up and maintain soil organic matter will help eliminate many problems, or at least make them easier to manage.
As of the writing of this edition, there are discussions around how glyphosate-based herbicides affect micronutrient availability. Glyphosate is the most frequently applied herbicide worldwide and, like soil organic matter, has chelating abilities. It is still an open debate whether this has a significant impact on plant micronutrient availability or affects soil, plant health or human health. However, there is no conclusive evidence that it is overall more harmful than the chemicals it replaces.
Potassium (K) is one of the N-P-K “big three” primary nutrients needed in large amounts, and in humid regions it is frequently not present in sufficient quantities for optimum crop yields. Deficiencies occur more readily when the entire crop is harvested and removed versus the grain only. Unlike N and P, K is more concentrated in stalks and stems that remain in the field as stover/straw if only the grain is harvested, thereby recycling most of the K for the next crop. K is generally available to plants as a cation, and the soil’s cation exchange capacity (CEC) is the main storehouse for this element for a given year’s crop. Potassium availability to plants is sometimes decreased when a soil is limed to increase its pH by one or two units. The extra calcium, as well as the “pull” on K exerted by the new cation exchange sites (see the next section, “Cation Exchange Capacity Management”), contributes to lower K availability. Problems with low K levels are usually easily dealt with by applying muriate of potash (potassium chloride), potassium sulfate or K-mag (potassium magnesium sulfate, also sold as Sul-Po-Mag or Trio). Manures also usually contain large quantities of K. Some soils have low amounts of CEC, such as sandy and sandy loams low in both organic matter and clay. But if the type of clay has low CEC, such as kaolinitic clays found in the Southeast, low CEC may make it impossible to store large amounts of readily K for plants to use. If a lot of fertilizer K is added at one time—an amount that may be reasonable for another soil—a significant portion may be leached below the root zone before plants can use it. In these situations, split applications of K may be needed. Since most complete organic fertilizers are low in K, organic growers with low CEC soils need to pay special attention to maintaining the K status of their soils.
Magnesium deficiency is easily corrected, if the soil is acidic, by using a magnesium (dolomitic) lime to raise the soil pH (see “Soil Acidity”). If K is also low and the soil does not need liming, potassium magnesium sulfate is one of the best choices for correcting a magnesium deficiency. For a soil that has sufficient K and is at a satisfactory pH, a straight magnesium source such as magnesium sulfate (Epsom salts) would be a good choice.
Calcium deficiencies are generally associated with low pH soils and soils with a low CEC. The best remedy is usually to lime and build up the soil’s organic matter. However, some important crops, such as peanuts, potatoes and apples, commonly need added calcium. Calcium additions also may be needed to help alleviate soil structure and nutrition problems of sodic soils or soils that have been flooded by seawater (see “Remediation of Sodic [Alkali] and Saline Soils”). In general, there will be no advantage to adding a calcium source, such as gypsum, if the soil does not have too much sodium, is properly limed and has a reasonable amount of organic matter. However, soils with very low aggregate stability may sometimes benefit from the extra salt concentration and calcium associated with surface gypsum applications. This is not a calcium nutrition effect but is a stabilizing effect of the dissolving gypsum salt. Higher soil organic matter and surface residues should do as well as gypsum to alleviate this problem.
Sulfur deficiency is common on coarse texture soils with low organic matter, in part because it is subject to leaching in the oxidized sulfate form (similar to nitrate). Some soil testing labs around the country offer a sulfur soil test. (Those of you who grow garlic should know that a good supply of sulfur is important for the full development of garlic’s pungent flavor.) Much of the sulfur in soils occurs as organic matter, so building up and maintaining organic matter should result in sufficient sulfur nutrition for plants. Sulfur deficiency is becoming more common in certain regions now that there is less sulfur air pollution, which previously originated from combustion of high-sulfur forms of coal. (Now it is captured in power plant exhaust scrubbers, and the residue is sold as gypsum.) In the Great Plains, on the other hand, irrigation water may contain sufficient quantities of sulfur to supply crop needs even though the soils are deficient in sulfur. And some fertilizers used for other purposes, such as potassium sulfate, potassium magnesium sulfate and ammonium sulfate, contain sulfur. Calcium sulfate (gypsum) also can be applied to remedy low soil sulfur. The amount used on sulfur-deficient soils is typically 15–25 pounds of sulfur per acre.
The risk for sulfur deficiency varies with the soil type, the crops grown on the soil, the manure history and the level of organic matter in the soil. A deficiency is more likely to occur on acidic, sandy soils; soils with low organic matter levels and high nitrogen inputs; and soils that are cold and dry in the spring, which decreases sulfur mineralization from soil organic matter. Manure is a significant supplier of sulfur, and manured fields are not likely to be S deficient; however, sulfur content in manure can vary.
—S. Place et al. (2007)
Zinc deficiencies occur with certain crops on soils low in organic matter, and in sandy soils or soils with a pH at or above neutral. Zinc problems are sometimes noted on silage corn when manure hasn’t been applied for a while. Zinc also can be deficient following topsoil removal from parts of fields as land is leveled for furrow irrigation. Cool and wet conditions may cause zinc to be deficient early in the season. Sometimes crops outgrow the problem as the soil warms up and organic sources become more available to plants. Zinc deficiencies are also common in other regions of the world, especially Sub-Saharan Africa, South and East Asia, and parts of Latin America. Applying about 10 pounds of zinc sulfate per acre (which contains about 3 pounds of zinc) to soils is one method used to correct zinc deficiencies. If the deficiency is due to high pH, or if an orchard crop is zinc deficient, a foliar application is commonly used. If a soil test before planting an orchard reveals low zinc levels, zinc sulfate should be applied.
Boron deficiencies occur most frequently on sandy soils with low organic matter and on alkaline/calcareous soils. It shows up in alfalfa when it grows on eroded knolls where the topsoil and organic matter have been lost. Deficiencies are common in certain regions with naturally low boron, such as in the Northwest maritime area, and in many regions in other parts of the world. Root crops seem to need higher soil boron levels than do many other crops. Cole crops, apples, celery and spinach are also sensitive to low boron levels. The most common fertilizer used to correct a boron deficiency is sodium tetraborate (about 15% boron). Borax (about 11% boron), a compound containing sodium borate, also can be used to correct boron deficiencies. On sandy soils low in organic matter, boron may be needed on a routine basis. Applications for boron deficiency are usually around 1–2 pounds of boron per acre. No more than 3 pounds of actual boron (about 27 pounds of borax) per acre should be applied at any one time; it can be toxic to some plants at higher rates.
Manganese deficiency, usually associated with soybeans and cereals grown on high-pH soils and on vegetables grown on muck soils, is corrected with the use of manganese sulfate (about 27% manganese). About 10 pounds of water-soluble manganese per acre should satisfy plant needs for a number of years. Up to 25 pounds per acre of manganese is recommended if the fertilizer is broadcast on a very deficient soil. Natural, as well as synthetic, chelates (at about 5% to 10% manganese) usually are applied as a foliar spray.
Iron deficiency occurs in blueberries when they are grown on moderate- to high-pH soils, especially a pH of over 6.5. Iron deficiency also sometimes occurs in soybeans, wheat, sorghum and peanuts growing on soil with a pH greater than 7.5. Iron (ferrous) sulfate or chelated iron is used to correct iron deficiency. Reducing plant stressors such as compaction and selecting more tolerant crop varieties are also ways of reducing iron deficiency damage to crops. In addition, research in Minnesota indicates that companion planting a small amount of oats (whose roots are able to mobilize iron) with soybeans reduces iron deficiency symptoms. Manganese and iron deficiencies are frequently corrected by adding inorganic salts in a foliar application.
Copper is another nutrient that is sometimes deficient in high-pH soils. It can also be deficient in organic soils (soils with 10–20% or more organic matter). Some crops—for example, tomatoes, lettuce, beets, onions and spinach—have a relatively high copper need. A number of copper sources, such as copper sulfate and copper chelates, can be used to correct a copper deficiency.
High-end fertilizer materials have been developed that combine many macro and micronutrients into a single product that can be applied as seed coatings, leaf sprays (foliar), directly to the soil or through fertigation systems, and they are especially of interest for high-value crops.
Cation Exchange Capacity (CEC) Management
The CEC in soils is due to well-humified (“very dead”) organic matter and clay minerals. The total CEC in a soil is the sum of the CEC due to organic matter and due to clays. In fine-textured soils with medium- to high-CEC clays, much of the CEC may be due to clays. Conversely, in sandy loams with little clay, or in some of the soils of the southeastern United States and of the tropics that contain clays with low CEC, organic matter may account for an overwhelming fraction of the total CEC. There are two practical ways to increase the ability of soils to hold nutrient cations such as potassium, calcium, magnesium and ammonium:
- Add organic matter by using the methods discussed in earlier chapters.
- If the soil is too acidic, use lime (see “pH Management”) to raise its pH to the high end of the range needed for the crops you grow.
One of the benefits of liming acid soils is increasing soil CEC. As the pH increases, so does the CEC of organic matter as well as some clay minerals. As hydrogen (H+) on humus is neutralized by liming, the site where it was attached now has a negative charge and can hold Ca++, Mg++, K+, etc.
Many soil testing labs will run CEC if asked. However, there are a number of possible ways to do the test. Some labs determine what the CEC would be if the soil’s pH was 7 or higher. They do this by adding the acidity that would be neutralized if the soil was limed to the current soil CEC. This is the CEC the soil would have at the higher pH but is not the soil’s current CEC. For this reason, some labs total the major cations actually held on the CEC (Ca++ + K+ + Mg++) and call it effectiveCEC. It is more useful to know the effective CEC—the actual current CEC of the soil—than CEC determined at a higher pH.
Estimating Organic Matter's Contribution to a Soil's CEC
The CEC of a soil is usually expressed in terms of the number of milliequivalents (me) of negative charge per 100 grams of soil. (The actual number of charges represented by one me is about 6 followed by 20 zeros.) A useful rule of thumb for estimating the CEC due to organic matter is as follows: for every pH unit above pH 4.5, there is 1 me of CEC in 100 grams of soil for every percent of organic matter. (Don’t forget that there will also be CEC due to clays.) SOM = soil organic matter.
Example 1: pH = 5 and 3% SOM → (5 – 4.5) x 3 = 1.5 me/100g
Example 2: pH = 6 and 3% SOM → (6 – 4.5) x 3 = 4.5 me/100g
Example 3: pH = 7 and 3% SOM → (7 – 4.5) x 3 = 7.5 me/100g Example 4: pH = 7 and 4% SOM → (7 – 4.5) x 4 = 10 me/100g
SOIL ACIDITY
Background
- pH 7 is neutral.
- Soils with pH levels above 7 are alkaline; those of less than 7 are acidic.
- The lower the pH, the more acidic is the soil.
- Soils in humid regions tend to be acidic; those in semiarid and arid regions tend to be around neutral or alkaline.
- Acidification is a natural process.
- Most commercial nitrogen fertilizers are acid forming, but many manures are not.
- Crops have different pH needs, probably related to nutrient availability or susceptibility to aluminum toxicity at low pH.
- Organic acids on humus and aluminum on the CEC account for most of the acid in soils.
Management
- Test soils regularly, every other year if possible, to track soil acidity changes and to make timely adjustments if needed.
- Use limestone to raise the soil pH. (If magnesium is also low, use dolomitic lime, which contains magnesium in addition to calcium.)
- Mix lime thoroughly into the plow layer.
- Spread lime well in advance of planting sensitive crops, if at all possible.
- If the lime requirement is high—some labs say greater than 2 tons, others say greater than 4 tons—consider splitting the application over two years.
- Reducing soil pH (making soil more acid) for acid-loving crops is best done using elemental sulfur (S).
Soil Acidity
Background
A soil’s pH (or acidity status) is critical information because it influences nutrient chemistry and availability, and directly influences plant growth. Many soils, especially in humid regions, were acidic before they were ever farmed. Leaching of bases from soils and the acids produced during organic matter decomposition combined to make these soils naturally acidic. As soils were brought into production and organic matter decomposed (mineralized), more acids were formed. In addition, the most commonly used N fertilizers acidify soil as their ammonium is either converted to nitrate or is taken up by plants. Generally 4–7 pounds of lime are required to neutralize the acid formed from each pound of N applied to soils. Fertilizers that supply all their N in the form of nitrate, however, do not acidify the soil. In fact, applying calcium nitrate or potassium nitrate can slightly raise soil pH.
Plants have evolved in specific environments, which in turn influence their needs as agricultural crops. For example, alfalfa originated in a semiarid region where soil pH was high; alfalfa requires a pH in the range of 6.5–6.8 or higher (see Figure 20.1 for common soil pH levels). But blueberries, which evolved under acidic conditions, require a low pH to provide needed iron (iron is more soluble at low pH). Other crops, such as peanuts, watermelons and sweet potatoes, do best in moderately acid soils in the range of pH 5–6. Most other agricultural plants do best in the range of pH 6–7.5.
Several problems may cause poor growth of acid-sensitive plants in low pH soils. Three common problems:
- aluminum and manganese are more soluble and can be toxic to plants;
- calcium, magnesium, potassium, phosphorus or molybdenum (especially needed for nitrogen fixation by legumes) may be deficient; and
- decomposition of soil organic matter is slowed and causes decreased mineralization of nitrogen.
The problems caused by soil acidity are usually less severe, and the optimum pH is lower, if the soil is well supplied with organic matter. Organic matter helps to make aluminum less toxic, and, of course, humus increases the soil’s CEC. Also, soil pH will not change as rapidly in soils that are high in organic matter. Soil acidification is a natural process that is accelerated by acids produced in soil by most nitrogen fertilizers. Soil organic matter slows down acidification and buffers the soil’s pH because it holds the acid hydrogen tightly. Therefore, more acid is needed to decrease the pH by a given amount when a lot of organic matter is present. Of course, the reverse is also true: more lime is needed to raise the pH of high-organic-matter soils by a given amount (see “Soil Acidity” box).
Limestone application helps create a more hospitable soil for acid-sensitive plants in many ways:
- by neutralizing acids
- by adding calcium in large quantities (because limestone is calcium carbonate, CaCO3)
- by adding magnesium in large quantities if dolomitic limestone is used (containing carbonates of both calcium and magnesium)
- by making molybdenum and phosphorus more available
- by helping to maintain added phosphorus in an available form
- by enhancing bacterial activity, including the rhizobia that fix nitrogen in legumes
- by making aluminum and manganese less soluble
Almost all the acid in acidic soils is held in reserve on the solids, with an extremely small amount active in the soil water. If all that we needed to neutralize was the acid in the soil water, a few handfuls of lime per acre would be enough to do the job, even in a very acid soil. However, tons of lime per acre are needed to raise the pH. The explanation for this is that almost all of the acid that must be neutralized in soils is “reserve acidity” associated with either organic matter or aluminum. And as the acid (H+) is removed from organic matter, new CEC sites are created, increasing the soil’s ability to hold cations such as calcium and potassium. (It also works in reverse as soils are acidified: H+ strongly attaches to what had been CEC sites, removing their ability to hold onto calcium, magnesium, potassium and ammonium.)
pH Management
Increasing the pH of acidic soils is usually accomplished by adding ground or crushed limestone. Three pieces of information are used to determine the amount of lime that’s needed.
SOIL SAMPLING FOR pH
Traditionally soils have been sampled to 6 inches or deeper, depending on the depth of plowing. But for farmers using conservation tillage, especially no-till, the top few inches can become acidic while the zone below is largely unaffected. Over time, acidity will work its way deeper. But it is important to catch a significant pH decline early, when it’s easy to correct. Therefore, in no-till fields it’s best to follow pH changes in the top 2 or 3 inches.
Conversely, old soils in tropical regions often have high acidity in the lower soil regions, and a sample from deeper depths may be warranted.
- What is the soil pH? Knowing this and the needs of the crops you are growing will tell you whether lime is needed and what target pH you are shooting for. You need to use lime if the soil pH is much lower than the pH needs of the crop. But the pH value doesn’t tell you how much lime is needed.
- What is the lime requirement needed to change the pH to the desired level? (The lime requirement is the amount of lime needed to neutralize the hydrogen, as well as the reactive aluminum, associated with organic matter as well as clays.) Soil testing laboratories use a number of different tests to estimate soil lime requirements. Most give the results in terms of tons per acre of agricultural grade limestone to reach the desired pH.
- Is the limestone you use very different from the one assumed in the soil test report? The fineness and the amount of carbonate present govern the effectiveness of limestone, or, how much it will raise the soil’s pH. If the lime you will be using has an effective calcium carbonate equivalent that’s very different from the one used as the base in the report, the amount applied may need to be adjusted upward (if the lime is very coarse or has a high level of impurities) or downward (if the lime is very fine, is high in magnesium, and contains few impurities).
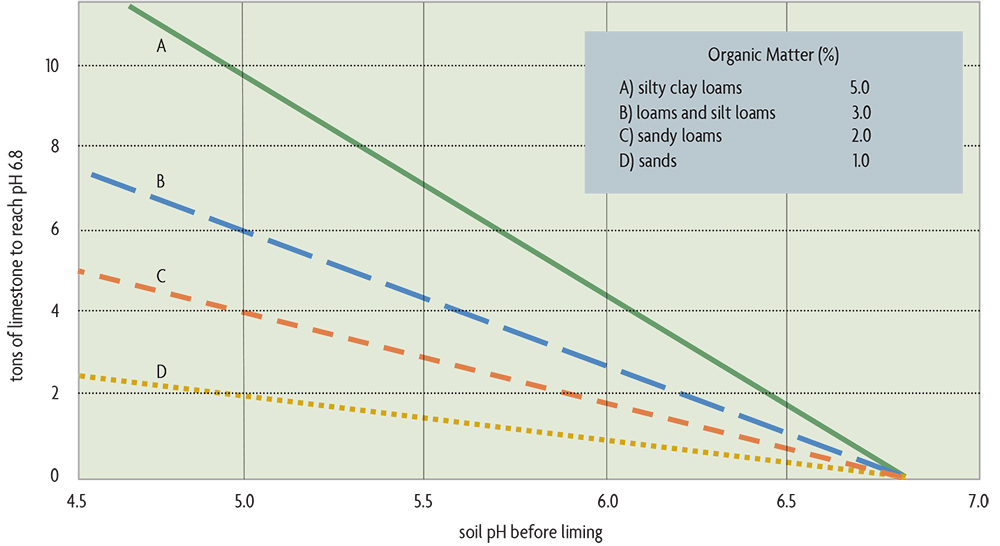
Soils with more clay and more organic matter need more lime to change their pH (see Figure 20.2). Although organic matter and clays buffer the soil against pH decreases, they also buffer against pH increases when you are trying to raise the pH with limestone. Most states recommend a soil pH of around 6.8 only for the most sensitive crops, such as alfalfa, and of about 6.2–6.5 for many of the clovers. As pointed out above, most of the commonly grown crops do well in the range of pH 6–7.5.
Soil testing labs usually use the information you provide about your cropping intentions and integrate the three issues when recommending limestone application rates. (See the discussion under “pH Management” on the three pieces of information needed.) Laws govern the quality of limestone sold in each state. The limestone recommendations given by soil testing labs meet the minimum state standard.
There are other liming materials in addition to limestone. One commonly used in some parts of the United States is wood ash. Ash from a modern airtight wood-burning stove may have a fairly high calcium carbonate content (80% or higher). However, ash that is mainly black—indicating incompletely burned wood—may have as little as 40% effective calcium carbonate equivalent. On the other hand, the char may provide other benefits to soil (see biochar discussion in Chapter 2). Lime sludge from wastewater treatment plants and fly ash sources may be available in some locations. Normally, minor sources like these are not locally available in sufficient quantities to put much of a dent in the lime needs of a region. Because they might carry unwanted contaminants to the farm, be sure that you test any new byproduct source of lime through an accredited laboratory for trace elements as well as metals and other potential toxins.
Liming and soil structure. Soil aggregation may show some improvements when applying calcium carbonate to soils that are relatively high in magnesium. The higher ionic strength of calcium pulls clay particles together better than magnesium. Conversely, when using dolomitic limestone, magnesium is added and may have the reverse effect (although the magnesium may be beneficial to the crop if it is deficient).
But liming is causing concerns when combined with no-till in the acidic cerrado (savanna) soils in Brazil, a productive region that has become a major global exporter of soybeans, beef and poultry. In these deeply weathered and highly oxidized soils, the structural degradation can be especially pronounced because the formation of aggregates under the naturally low pH of these soils results from high concentrations of Al3+ (which has high ionic strength) and dispersed organic matter. The negative charges on organic matter bind to the positive charges of oxides, and Al ions form bridges between organic matter and the minerals. However, liming raises the soil pH, which results in negative charges on soil particles and repulsion between them. In addition, high concentrations of calcium salts remove Al3+ from negatively charged sites within the soil, which reduces plant toxicity but also results in further dispersion of the clays and loss of aggregation. Under no-till, the dispersed clay can move with water to lower layers and cause dense and hard soils.
“Overliming” injury. Sometimes problems are created when soils are limed, especially when a very acidic soil has been quickly raised to high pH levels. Decreased crop growth because of “overliming” injury is usually associated with a lowered availability of phosphorus, potassium or boron, although zinc, copper and manganese deficiencies can be produced by liming acidic sandy soils. If there is a long history of the use of triazine herbicides, such as atrazine, liming may release these chemicals and kill sensitive crops.
Need to lower the soil’s pH? You may want to add acidity to the soil when growing plants that require a low pH. This is probably only economically possible for blueberries and is most easily done with elemental sulfur, which is converted into sulfuric acid by soil microorganisms over a few months to years (depending on the fineness of the material applied). For the examples in Figure 20.2, the amount of sulfur needed to drop the pH by one unit would be approximately 3/4 ton per acre for silty clay loams, 1/2 ton per acre for loams and silt loams, 600 pounds per acre for sandy loams, and 300 pounds per acre for sands. Sulfur should be applied the year before planting blueberries. Alum (aluminum sulfate) may also be used to acidify soils. About six times more alum than elemental sulfur is needed to achieve the same pH change. If your soil is calcareous—usually with a pH over 7.5 and naturally containing calcium carbonate—don’t even try to decrease the pH. Acidifying material will have no lasting effect on the pH because it will be fully neutralized by the soil’s lime.
Remediation of Sodic (Alkali) and Saline Soils
The origin and characteristics of saline and sodic soils were discussed at the end of Chapter 6. There are a number of ways to deal with saline soils that don’t have shallow salty groundwater. One is to keep the soil continually moist. For example, if you use drip irrigation with low-salt water plus a surface mulch, the salt content will not get as high as it would if allowed to concentrate when the soil dries. Another way is to grow crops or varieties of crops that are more tolerant of soil salinity. Saline-tolerant plants include barley, bermuda grass, oak, rosemary and willow. However, the only way to get rid of the salt is to add sufficient water to wash it below the root zone. If the subsoil does not drain well, drainage tiles might need to be installed to lower the water table and remove the salty water leached from the soil. (However, this means that a high-salt water is being discharged into a ditch and may harm downstream water quality. See also Chapter 17.) The amount of water needed to do this is related to the salt content of the irrigation water, expressed as electrical conductivity (ECw), and the salt content desired in the drainage water, expressed as electrical conductivity (ECdw). The amount of water needed can be calculated using the following equation:
Water needed = (amount of water needed to saturate soil) x (ECw/ECdw)
The amount of extra irrigation water needed to leach salts is also related to the sensitivity of the plants that you’re growing. For example, sensitive crops like onions and strawberries may have twice the leaching requirement of moderately sensitive broccoli or tomatoes. Drip irrigation uses relatively low amounts of water, so lack of leaching may cause salt buildup even for moderately saline irrigation sources. This means that the leaching may need to occur during the growing season, but care is needed to prevent nitrate leaching below the root zone.
For sodic soils, a calcium source is added, usually gypsum (calcium sulfate). The calcium replaces sodium held by the CEC. The soil is then irrigated so that the sodium can be leached deep into the soil. Because the calcium in gypsum easily replaces the sodium on the CEC, the amount of gypsum needed can be estimated as follows: for every milliequivalent of sodium that needs to be replaced to 1 foot, about 2 tons of agricultural-grade gypsum is needed per acre. Gypsum is not a liming source and may actually decrease the high pH of sodic soils (commonly pH 8.4 or higher). Adding gypsum to non-sodic soils doesn’t help physical properties if the soil is properly limed, except for those soils that contain easily dispersible clay and that are also low in organic matter. Sodic soils can also occur after major coastal flooding events, as the seawater washes a lot of sodium chloride through the flooded soil. This is especially of concern after hurricanes (typhoons), tsunamis or other unusual storm surges. The same remediation method, adding gypsum (or lime when the soil is naturally acidic), helps restore the soils in those cases.
Chapter 20 Sources
Hanson, B.R., S.R. Grattan and A. Fulton. 1993. Agricultural Salinity and Drainage. Publication 3375. University of California, Division of Agriculture and Natural Resources: Oakland, CA.
Havlin, J.L., J.D. Beaton, S.L. Tisdale and W.I. Nelson. 2005. Soil Fertility and Fertilizers. Pearson/ Prentice Hall: Upper Saddle River, NJ.
Kaiser, D.E. and P.R. Bloom. Managing Iron Deficiency Chlorosis in Soybean, University of Minnesota Extension. Accessed December 4, 2019 at https://extension.umn.edu/crop-specific-needs/managing-iron-deficiency-chlorosis-soybean#reduce-plant-stress-1074262.
Magdoff, F.R. and R.J. Bartlett. 1985. Soil pH buffering revisited. Soil Science Society of America Journal 49: 145–148.
Nunes, M.R., A.P. da Silva, C.M.P. Vaz, H.M. van Es and J.E. Denardin. 2018. Physico-chemical and structural properties of an Oxisol under the addition of straw and lime. Soil Sci. Soc. Am. J. 81:1328–1339.
Peech, M. 1961. Lime Requirement vs. Soil pH Curves for Soils of New York State. Mimeographed. Cornell University Agronomy Department: Ithaca, NY.
Pettygrove, G.S., S.R. Grattan, T.K. Hartz, L.E. Jackson, T.R. Lockhart, K.F. Schulbach and R. Smith. 1998. Production Guide: Nitrogen and Water Management for Coastal Cool-Season Vegetables. Publication 21581. University of California, Division of Agriculture and Natural Resources: Oakland, CA.
Place, S., T. Kilcer, Q. Ketterings, D. Cherney and J. Cherney. 2007. Sulfur for Field Crops. Agronomy Fact Sheet Series no. 34. Cornell University Cooperative Extension: Ithaca, NY. Rehm, G. 1994. Soil Cation Ratios for Crop Production. North Central Regional Extension Publication 533. University of Minnesota Extension Service: St. Paul, MN.
